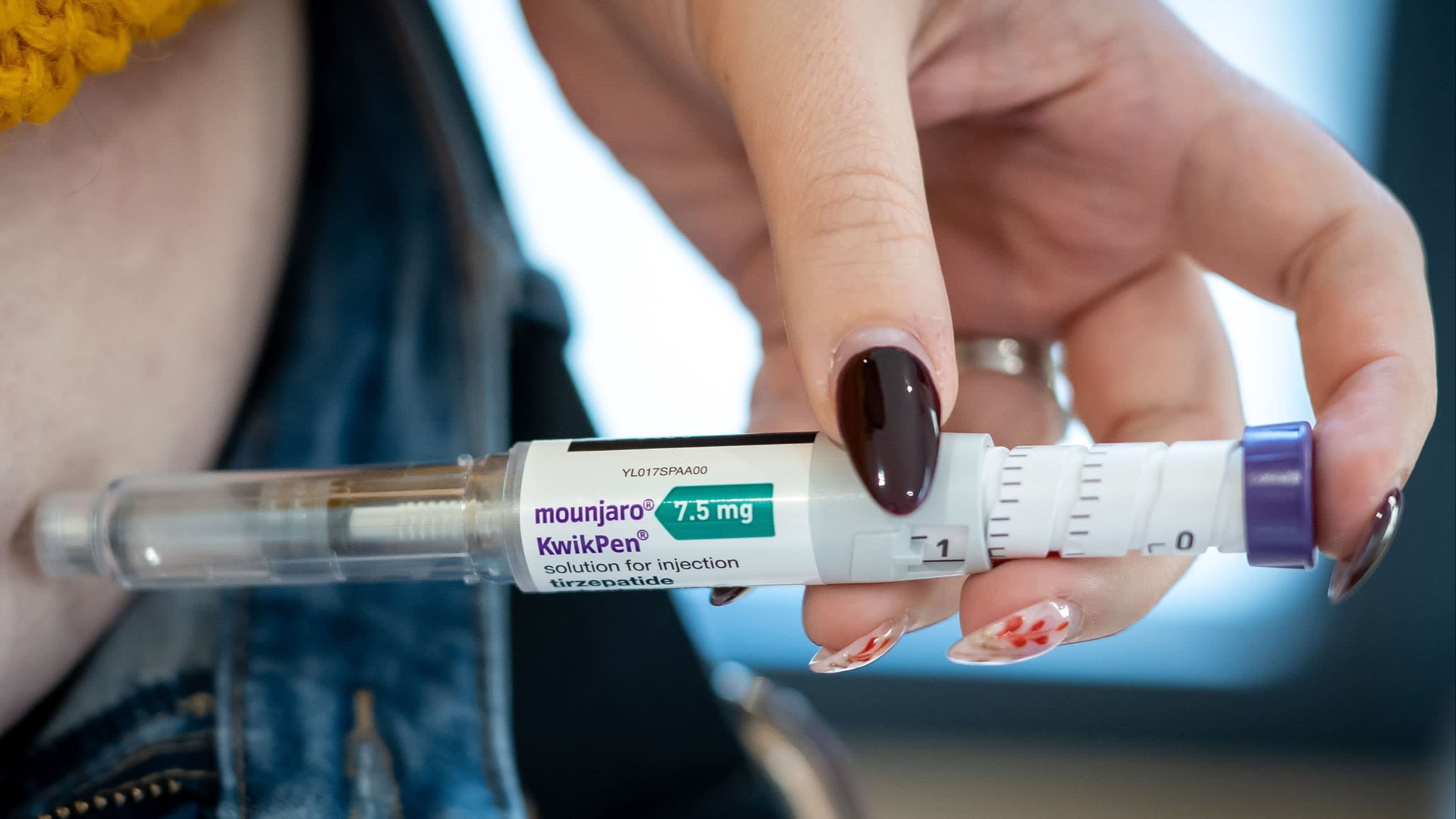
The National Health Service (NHS) in England is significantly tightening the eligibility criteria for the latest generation of weight-loss injections, a move that signals a growing tension between breakthrough medical innovation and the fiscal realities of public healthcare. As Integrated Care Boards (ICBs) across the country grapple with ballooning deficits and unprecedented demand, the rollout of glucagon-like peptide-1 (GLP-1) receptor agonists—most notably Novo Nordisk’s Wegovy—has hit a regulatory and financial bottleneck. This shift in policy marks a pivotal moment in the UK’s public health strategy, highlighting the challenges of funding transformative but expensive treatments for chronic conditions within a centralized budget.
The decision to narrow the gateway for these treatments comes despite clinical evidence suggesting that drugs like semaglutide (Wegovy) and tirzepatide (Mounjaro) are highly effective in reducing body mass and mitigating the risks of obesity-related comorbidities, such as Type 2 diabetes, cardiovascular disease, and certain cancers. However, the sheer scale of the obesity crisis in the United Kingdom, where nearly two-thirds of adults are classified as overweight or obese, has created a demand curve that the NHS’s current infrastructure is ill-equipped to handle. By raising the threshold for access, local health authorities are effectively rationing a treatment that was initially hailed as a panacea for the nation’s waistline.
The economic burden of obesity in the United Kingdom is staggering. Recent estimates suggest that obesity-related illnesses cost the NHS approximately £6.5 billion annually, a figure projected to rise as the population ages and BMI levels remain elevated. When considering the wider socio-economic impact—including lost productivity, increased welfare payments, and premature mortality—the total cost to the UK economy is estimated to be upwards of £58 billion per year. From a purely economic perspective, investing in weight-loss medications appears to be a sound long-term strategy. Proponents argue that the upfront cost of the injections is far outweighed by the long-term savings generated by preventing expensive surgeries and lifelong chronic disease management.
Despite this "preventative" logic, the immediate liquidity crisis within the NHS has forced a more conservative approach. Integrated Care Boards, the local bodies responsible for planning and delivering healthcare services, are facing a collective funding gap that has reached billions of pounds. In this climate, the introduction of a high-cost, high-volume medication like Wegovy presents a significant "budget impact" risk. Unlike specialized treatments for rare diseases, weight-loss drugs target a demographic comprising millions of citizens. Even with the confidential discounts negotiated between the government and pharmaceutical manufacturers, the cumulative cost of treating a significant portion of the eligible population could potentially destabilize local health budgets.
Furthermore, the tightening of criteria is not merely a matter of drug costs but also one of clinical infrastructure. The National Institute for Health and Care Excellence (NICE) initially recommended that Wegovy be prescribed within "specialist weight management services," often referred to as Tier 3 or Tier 4 services. These services are multidisciplinary, involving dieticians, psychologists, and specialized physicians. However, these services are notoriously underfunded and subject to a "postcode lottery," with some regions offering robust support while others have waitlists stretching into years. By tightening the criteria, health officials are attempting to prevent these already overstretched services from being overwhelmed by a surge of new referrals.
The pharmaceutical landscape itself adds another layer of complexity to this issue. The global market for GLP-1 drugs has exploded, with Novo Nordisk and Eli Lilly emerging as the dominant players in a sector that some analysts predict could be worth $100 billion by 2030. This surge in demand has led to persistent global supply shortages. Novo Nordisk has struggled to ramp up production of Wegovy fast enough to meet international demand, particularly as the drug gained viral popularity in the United States. In the UK, the limited supply has forced the government to prioritize those with the highest clinical need, often focusing on patients with a BMI over 35 and at least one weight-related comorbidity, or those who have failed all other interventions.
From a global perspective, the UK’s cautious rollout contrasts sharply with the United States, where the "wellness" and "longevity" markets have embraced GLP-1s with fervor. In the US, access is largely determined by private insurance coverage or the ability to pay out-of-pocket, leading to a rapid, if unequal, uptake. In contrast, European nations with socialized or highly regulated healthcare systems, such as France and Germany, have been more measured, often limiting reimbursement to those with severe obesity or related metabolic disorders. The UK is currently navigating a middle path, attempting to balance universal access with the cold reality of a fixed treasury allocation.
The economic impact analysis of this rationing is multifaceted. On one hand, restricting access may protect the NHS’s immediate cash flow. On the other hand, it may exacerbate existing health inequalities. Obesity disproportionately affects lower-income communities in England, where access to fresh food and exercise facilities is often limited. By making the "jab" harder to obtain through the NHS, the government risks creating a two-tier system: one where the affluent can access the drugs via private prescriptions (which can cost several hundred pounds a month), while the most vulnerable populations remain trapped in a cycle of poor health and economic inactivity.
This leads to the broader debate regarding the UK’s workforce and economic growth. The British government has recently emphasized the need to reduce the number of people on long-term sickness benefits to stimulate the economy. Given that obesity is a leading cause of musculoskeletal issues and metabolic diseases that keep people out of work, some economists argue that the NHS should be expanding, not restricting, access to weight-loss treatments as a form of labor-market intervention. If a £200-a-month injection can return a person to the workforce and reduce their reliance on state benefits, the return on investment (ROI) for the taxpayer is substantial.
However, the "medicalization" of obesity also draws criticism from public health experts who argue that drugs are a sticking plaster for a systemic problem. They contend that the focus should remain on "upstream" interventions, such as sugar taxes, stricter regulations on ultra-processed foods, and urban planning that encourages physical activity. There is a fear among some policy-makers that a heavy reliance on GLP-1s will diminish the political will to tackle the environmental and commercial determinants of obesity. This philosophical divide often informs the stringency of the criteria set by local health boards, who must weigh the benefits of a pharmaceutical solution against the need for holistic lifestyle changes.
As the NHS moves forward, the criteria for these "weight-loss jabs" are likely to remain a flashpoint for debate. The introduction of Eli Lilly’s Mounjaro, which clinical trials suggest may be even more effective than Wegovy, could introduce much-needed competition into the market, potentially driving down prices and easing supply constraints. Furthermore, the development of oral versions of these drugs—pills rather than injections—could simplify the delivery model, removing the need for some of the expensive specialist infrastructure currently required.
For now, the tightening of rules in large parts of England serves as a sobering reminder of the limitations of a public healthcare system in an era of high-cost innovation. While the medical community continues to marvel at the efficacy of GLP-1s, the accountants at the NHS are left with the unenviable task of deciding who is "heavy enough" to warrant the investment. As the gap between medical possibility and fiscal reality widens, the resolution will likely require not just more funding, but a fundamental redesign of how the UK manages the intersection of pharmaceutical advancement, public health, and economic productivity. The evolution of this policy will be closely watched by global healthcare providers and pharmaceutical giants alike, as it represents a microcosm of the 21st-century challenge: how to pay for the cures we have finally discovered.